FAQs – QuickSwitch™ for MHC-peptide exchange
General
How many peptides can be exchanged and how many samples can be performed using 1 kit?
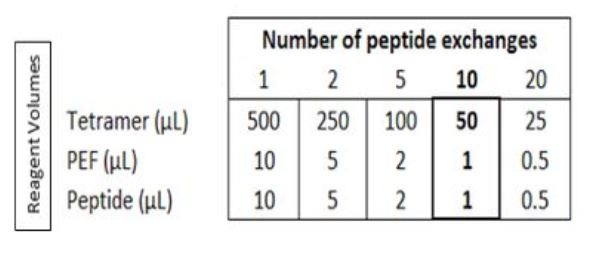
Each QuickSwitch™ kit is designed for 10 peptide exchange reactions. Therefore, approximately 45 μL of tetramer is available for staining after peptide exchange. Therefore, for each peptide, you can have 5 stainings using 9 μL of the resulting tetramer and 90 μL of cell suspension. Depending on the number of peptides to test, the volumes of the reagents will change as described in the table below:
If you are interested in screening a large library set of peptides, please contact us to learn more.
What is the difference between QuickSwitch™ kit vs. QuickSwitch™ Quant kit?
TB-7400 (Quant version) has extra components to quantify the binding affinity. TB-7401 (Non-Quant version) is a simple peptide exchange kit, where it is not possible to determine the binding affinity of your peptide. If you already know or are confident of the binding affinity of your peptide of interest and don’t need to quantify the binding affinity, then you may prefer to use the Non-Quant version of the QuickSwitch™ Kit.
What is the best fluorochrome?
Phycoerythrin (PE) is the fluorochrome of choice because it is brighter than Allophycocyanin (APC), followed by Brilliant Violet 421 (BV421). With our experience, APC tetramers can display more non-specific binding than the 2 other fluorochromes and sometimes have a propensity to aggregate. BV421 tetramers display the least amount of non-specific staining, probably because this fluorochrome is smaller and non-protein based.
What is the peptide concentration and purification needed for each antigen?
For MHC class I QuickSwitch™, a final 20 μM exchanging peptide concentration is sufficient for obtaining an 80-90% peptide exchange. For some lower affinity peptides, a higher concentration 100-200 μM may be more appropriate. For MHC class II QuickSwitch™, a final 1 mM concentration is advised but high affinity peptides can be exchanged with 100 μM or even 10 μM final concentrations. Thus, it is up to the user to test multiple exchanging peptide concentrations and determine which concentration is optimal.
What is the exiting peptide that come with the QuickSwitch™ tetramer ?
The exiting peptide is absent from any human, mouse, virus, bacteria, etc. The sequence is confidential.
How should I design the higher affinity peptide for peptide exchange using QuickSwitch™? What is the best size of peptide sequence to use? Is there any specific requirement or limitation for peptide purity?
It is up to the customer to use a prediction tool or software for MHC-peptide binding affinity predictions. We recommend peptides that are 8-11 aa long for MHC class I. For Class II you may go up to 25 aa. The minimum quantity of peptide needed is 100 μg. The peptides purity should be >90%.
What peptide exchange percentage is acceptable for use in subsequent tetramer staining assays? 50%, 70% or higher?
We recommend at least 75 % peptide exchange rate and higher because this corresponds to a trimer, the optimal configuration for cell binding. However it is possible that some low MHC binding peptides might generate MHC/peptide complexes that could bind to TcRs with a reasonable affinity. To ascertain their specific binding, it is a good idea to use in parallel tetramers made with irrelevant peptides of similar MHC binding affinity.
Researchers should not go below 55% peptide exchange.
Referring to the datasheet URL: https://blog.mblintl.com/hubfs/15.17.1.8-Quickswitch-Quant-H-2-kb-ve3_rev1909.pdf (page 4), there are 6 peptide samples tested for exchange efficiency and only one (sample E) is FALSE. “FALSE” means that the MFI signal is higher than the original tetramer, suggesting a negative peptide exchange. This phenomenon sometimes happens with highly hydrophobic peptides that aggregate tetramers and therefore cause high MFI signals.
What is your suggestion for the working concentration of lower affinity peptides using peptide exchange without tetramer aggregation?
For low affinity MHC binding peptides, increasing the final peptide concentration from 20 μM to 100 or 200 μM will increase the rate of peptide exchange and often result in peptide exchange rates equal or superior to 75%. In the case of hydrophobic peptides, high peptide concentrations may lead to tetramer aggregation. However, this is not an issue for most peptides.
What is the shelf life of resulting custom tetramer?
The shelf life of tetramer may be 3-12 months when stored at 2-40C. Primarily, tetramer stability depends on the peptide affinity to the MHC. However, peptide exchanged tetramers are surrounded by a high peptide concentration, which stabilizes the MHC/peptide complex. Therefore, peptide affinity is irrelevant in this context. The only potential problem could be peptide polymerization or deamidation of some critical amino acids, thereby reducing tetramer stability.
Can I use biotinylated antibodies with tetramers?
Please don’t use biotinylated antibodies in tetramer staining as they could bind to tetramers and contribute to some non-specific binding. Therefore, we do not recommend using any biotinylated antibodies or streptavidin conjugated reagents while working with tetramers.
Do I need to use KT15 clone CD8 ab when I use the H-2Kb Allele QuickSwitch™ kit?
The anti-mouse CD8 mAb KT15 does not interfere with tetramer H-2 Kb tetramer binding to TcRs, whereas most anti CD8 mAbs increase the non-specific binding of such tetramers. Therefore, we strongly recommend to use the KT15 mAb. https://products.mblintl.com/products/k0227-4/
Do we need to desalt the tetramer after peptide exchange?
In most cases, this is not necessary. It may be needed before staining with multiple peptide exchanged tetramers to prevent high affinity peptides to outcompete lower affinity ones.
Can I use QuickSwitch™ non-exchange tetramer as negative control?
Please do not use the non-exchanged QuickSwitch™ tetramer as negative control. Instead, use the tetramer obtained by peptide exchange with the reference peptide for your negative control.
Do all QuickSwitch™ kits contain the same capture beads?
All Human Class I Tetramer QuickSwitch™ kits use the same capture beads. They are different than the beads used for Mouse QuickSwitch™ Tetramer kits. Human Class II QuickSwitch™ Tetramer kits use different capture beads from human and mouse Class I kits.
I'm using the same peptide as I used with a previous exchange, why is my exchange rate not as high?
There could be several different issues. It is known that some peptide sequences are less stable than others. For example, your peptide may contain cysteines. This means that if they are too old or have been exposed for too long to oxygen, they will have the propensity to make internal or inter peptide disulfide bonds. Some amino acids such as asparagine and glutamine can undergo deamidation which consists of the loss of the amide functional group from side chains or their conversion to another functional group. Therefore, it is crucial to work (if possible) with recently made peptides to avoid these modifications.
Please contact our technical support team for further support regarding your unique experiments
Does the Human Class I QuickSwitch™ tetramer contain the α3 mutation?
Yes, it contains the α3 mutation.
I don't have a plate magnet. Can I still use your QuickSwitch™ kits?
Yes, by centrifugating the conical well plate at 1,000 g for 5 minutes, beads can be pelleted. Then, you can safely flick the plate to remove supernatants. In short, you can use the same centrifugation protocol as for staining cells. However, we recommend that pelleting beads with a plate magnet is preferential because it limits the risk of bead clumping.
Are there procedure differences between the kits?
Yes, please be sure to read the product data sheet pertaining to the specific allele before beginning experiments as there are procedural differences depending on the allele you are working with.